
Quality Assurance (QA) is essential for ensuring product quality, compliance, and robust operations. Our QA Benchmarking, developed over several years in collaboration with leading pharmaceutical companies and launched in Spring 2023, is the first benchmarking that holistically and systematically evaluates the performance and maturity of QA‑related processes.

Participants receive:
We evaluate your QA organization across 15 dedicated QA process fields, grouped into three categories:
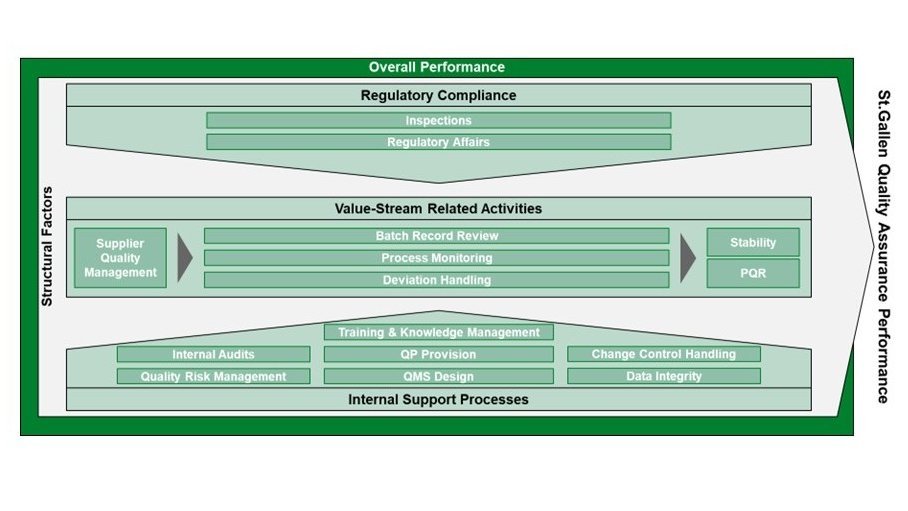
Each process field is assessed through a dedicated KPI set (performance) and qualitative enabler questions (maturity), enabling a holistic understanding of strengths, gaps, and successful practices. We additionally consider structural factors to define a meaningful peer group and ensure robust comparisons.
The QA Benchmarking was developed together with leading pharmaceutical companies to ensure relevance and applicability. It was officially launched in Spring 2023.
The project is fully financed by participating companies. This ensures independence from individual sponsors and guarantees high practical relevance.
The benchmarking project is conducted in accordance with the International Benchmarking Code of Conduct, which ensures that all participants behave in a fair and responsible manner. Company data is treated with the utmost confidentiality. Company-specific information may not be used without the prior consent of the company concerned. The institute reserves the right to use the project results in summarized and anonymized form for publication.