
Lean Lab initiatives and increasing QC complexity require a clear view of performance and maturity. Our QC Lab Benchmarking, based on the proven St.Gallen OPEX approach and supported by a global database of more than 190 QC labs, shows you where your lab stands and which factors enable top performance.
Participants receive:
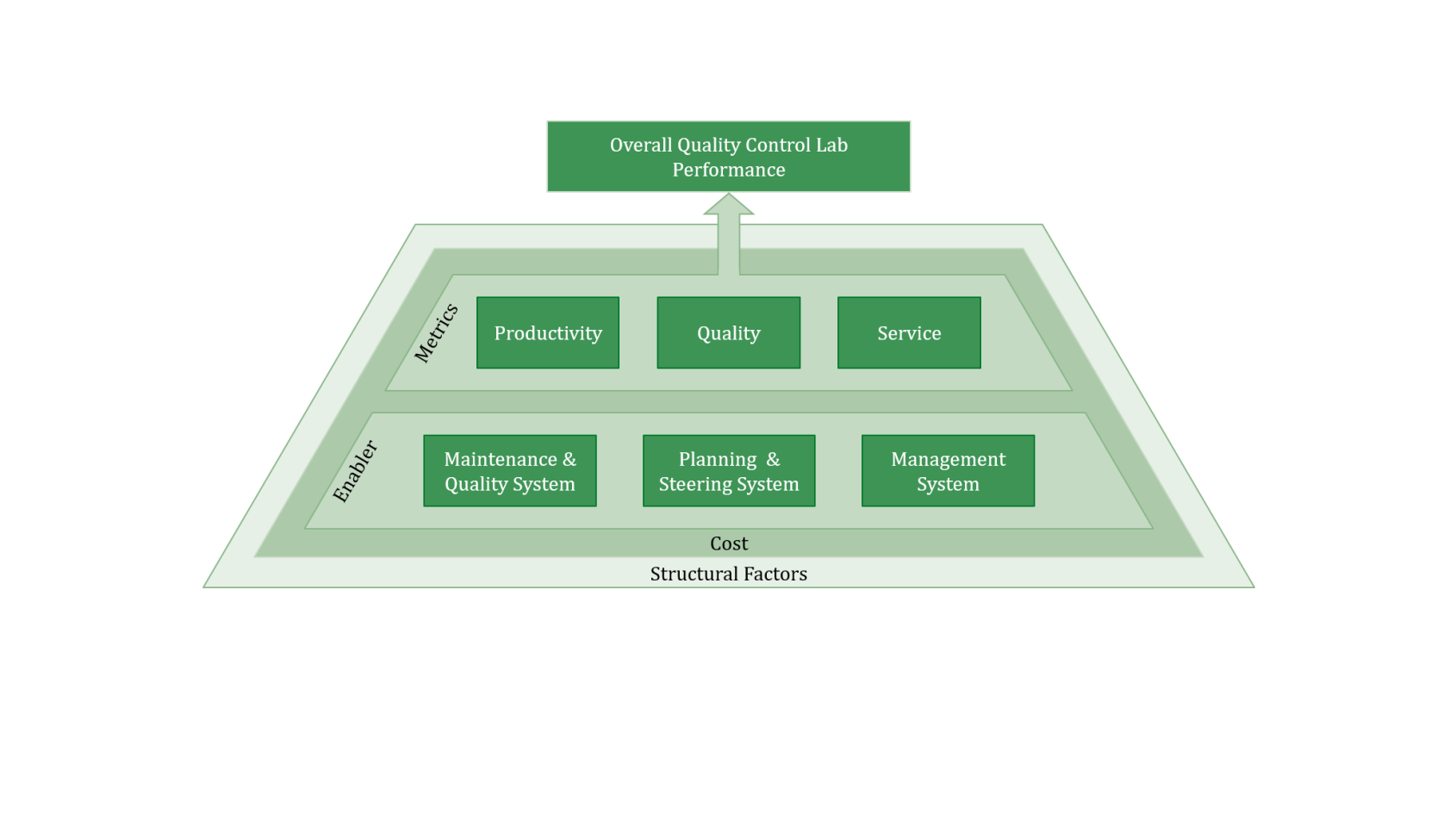
We evaluate your QC lab across three core performance dimensions:
And across three central enabler categories:
We additionally consider costs and structural factors, e.g., the size of the lab and different test categories.
The QC Lab Benchmarking was developed in 2016 together with leading pharmaceutical companies to ensure feasibility and practical relevance. Our database includes:
The project is fully financed by participating companies. This ensures independence from individual sponsors and guarantees high practical relevance.
The benchmarking project is conducted in accordance with the International Benchmarking Code of Conduct, which ensures that all participants behave in a fair and responsible manner. Company data is treated with the utmost confidentiality. Company-specific information may not be used without the prior consent of the company concerned. The institute reserves the right to use the project results in summarized and anonymized form for publication.